Defying Convention: New Approaches to Realizing Artificial Photosynthesis—Kazuhiko Maeda
Science Tokyo Faces vol. 001
Named Highly Cited Researcher by Clarivate for Four Consecutive Years
Artificial photosynthesis is the use of technology to mimic natural photosynthesis. Japan has been a leader in this advancing field since the discovery of the Honda-Fujishima effect in 1967 by Ken-ichi Honda (professor emeritus at The University of Tokyo) and Akira Fujishima (special university professor emeritus at The University of Tokyo and professor emeritus at Tokyo University of Science). Building on this legacy, Professor Kazuhiko Maeda at the Department of Chemistry, School of Science is defying conventional approaches to develop highly efficient photocatalysts. His contributions have earned international recognition, with Clarivate naming him a Highly Cited Researcher in Chemistry for four consecutive years starting in 2018.
What is “artificial photosynthesis“?
Photosynthesis is a process performed by plants and algae that is thought to have originated about 3.5 billion years ago. Photosynthesis is the process (chemical reaction) in which oxygen and organic compounds, such as sugars and starches, are produced from water and carbon dioxide using the energy of sunlight.
If this reaction could be reproduced artificially, it would be possible to create hydrogen from water using the limitless sunlight energy on Earth and convert carbon dioxide, a cause of global warming, into useful hydrocarbons, which would contribute to achieving a carbon-neutral society. Performing photosynthesis with human technology is referred to as “artificial photosynthesis.“
Professor Kazuhiko Maeda explained, “During my third year at university in 2001, I listened to a lecture by Professor Kazunari Domen, who was a leading researcher in artificial photosynthesis at the Tokyo Institute of Technology (currently a special contract professor at Shinshu University, a university professor at The University of Tokyo, and a professor emeritus at the Institute of Science Tokyo). I was thrilled by the idea that ’if artificial photosynthesis could be achieved, it would solve the world's environmental problems!’ This motivated me to become a researcher in this field. Of course, realizing this incredible function found in plants that took evolution 3.5 billion years to achieve would not be easy. However, just as we have developed airplanes with flight capabilities that far surpass those of birds, I believe we can achieve photosynthesis that surpasses that of plants.“

Researching “photocatalysts” that use visible light to split water
What exactly is the Honda-Fujishima effect, which triggered research into artificial photosynthesis? This is a phenomenon where water can be split into oxygen and hydrogen using energy from ultraviolet light instead of just electricity. It occurs when ultraviolet light is applied to an electrode that produces oxygen during water electrolysis, utilizing a compound called titanium dioxide. After this discovery, research into “photocatalysts“ like titanium dioxide, which can split water using light, significantly accelerated. Normally, when sunlight hits water, it is not split into oxygen and hydrogen. Titanium dioxide encourages this chemical reaction to split the water.
Photosynthesis in plants includes an “oxidation reaction“ that converts water to oxygen via sunlight, and a “reduction reaction“ that produces various organic compounds from carbon dioxide. Similarly, in the research and development of artificial photosynthesis, photocatalysts are being studied to promote these reactions, focusing on the “oxidation of water (oxygen production)“ and “reduction of carbon dioxide (hydrocarbon production)“ using sunlight.
Maeda went on to explain, “With the Honda-Fujishima effect, while titanium dioxide was discovered to have a photocatalyst function where it can split water, there is a major problem with this material. Titanium dioxide is only able to absorb ultraviolet light, which has a shorter wavelength compared to visible light (Figure 1). Only a few percent of sunlight is ultraviolet light. Even if it were possible to use all components of the limited ultraviolet band, calculations indicate that the energy conversion efficiency of sunlight with titanium dioxide would be less than 1%. For artificial photosynthesis to reach practical use, we would need to achieve an energy conversion efficiency of at least 5 to 10%. In this context, I was deeply impressed when, during a 2001 lecture, Professor Domen highlighted the existence of a photocatalyst that can absorb visible light. Since then, I have been studying photocatalysts capable of promoting water splitting using visible light for over 20 years.”
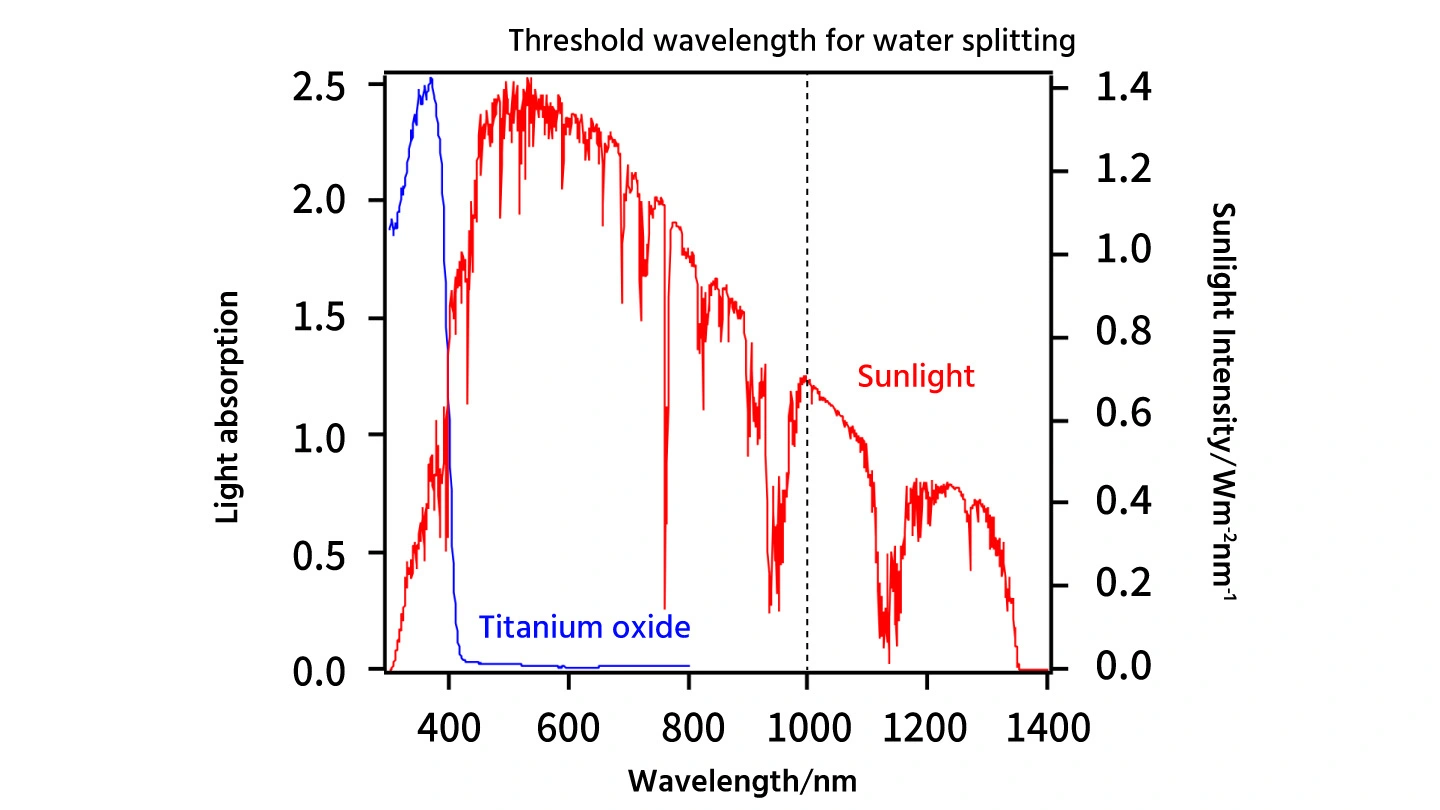
With titanium dioxide, only the ultraviolet range of sunlight (a few percent) can be utilized. To split water using sunlight, a substance capable of absorbing visible light, which is the main component of sunlight, is required. However, the smaller the light's energy (the longer the wavelengths), the more challenging it becomes to use it for artificial photosynthesis. *Red line indicates sunlight intensity (right axis). *Blue line indicates light absorption by titanium oxide (left axis).
Synthesizing a “mixed anion compound” that absorbs visible light
Indeed, Maeda is researching a photocatalyst known as a mixed-anion* compound. Titanium dioxide is a metal oxide where oxygen is bonded to titanium. Mixed-anion compounds are created by replacing some oxygen ions in metal oxides, such as titanium dioxide, with different anions.
”Titanium dioxide is a white powder. Its whiteness indicates it does not absorb visible light. However, when metal oxide is exposed to high-temperature ammonia gas (800°C to 1,000°C), oxynitrides (a mixed-anion compound of oxygen and nitrogen) of other colors are produced (Figure 2). The fact that these have color indicates that visible light of other colors is being absorbed. Among oxynitrides, there are compounds with different compositions and crystal structures, and new ones are still waiting to be discovered. There are a wide variety of mixed-anion compounds other than oxynitrides including oxysulfides and oxyfluorides. By examining these without being constrained by conventional ideas, it's possible to develop photocatalysts capable of absorbing a broader range of visible light.”
- Anion: A negatively charged ion, also called a negative ion.
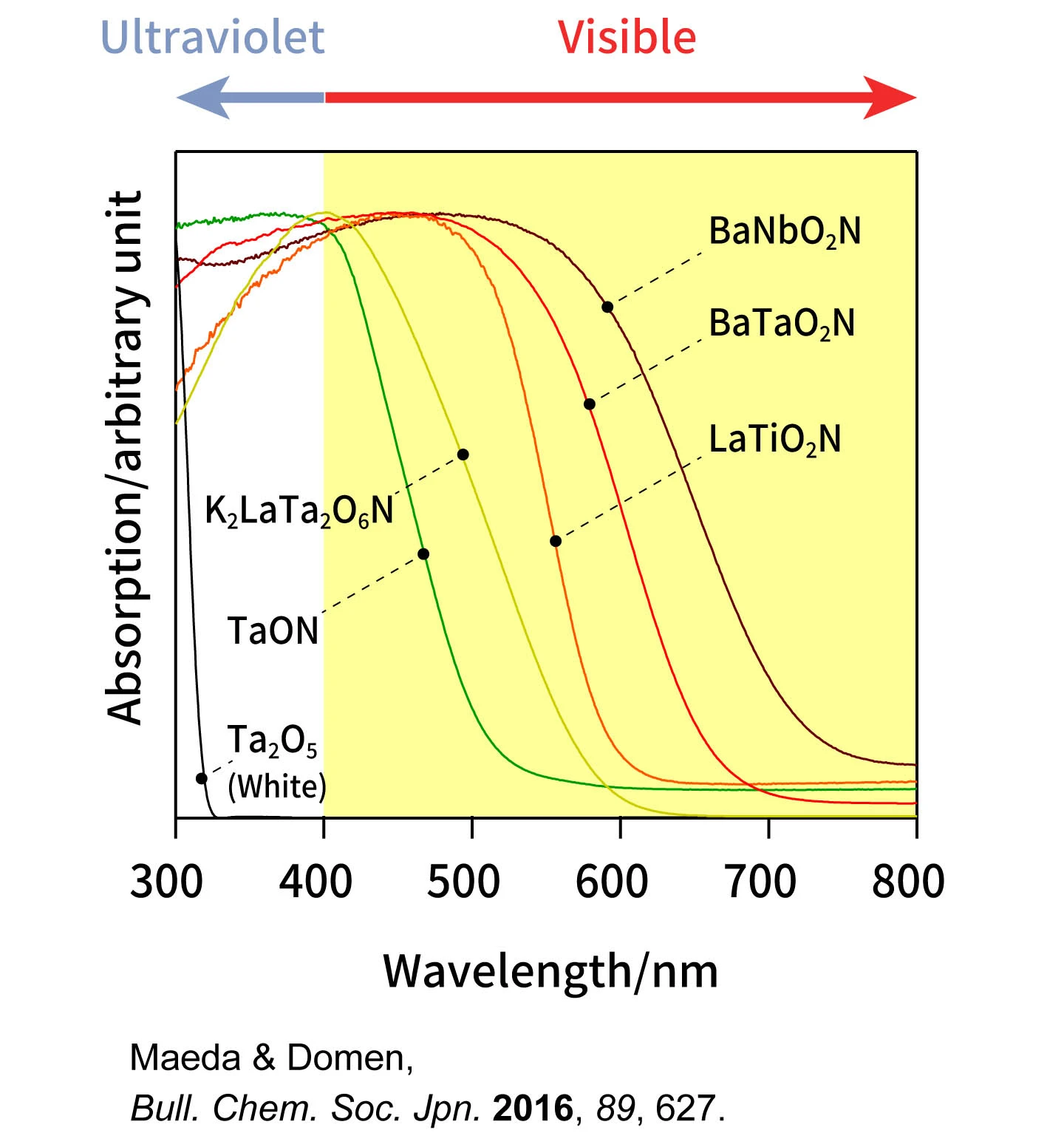
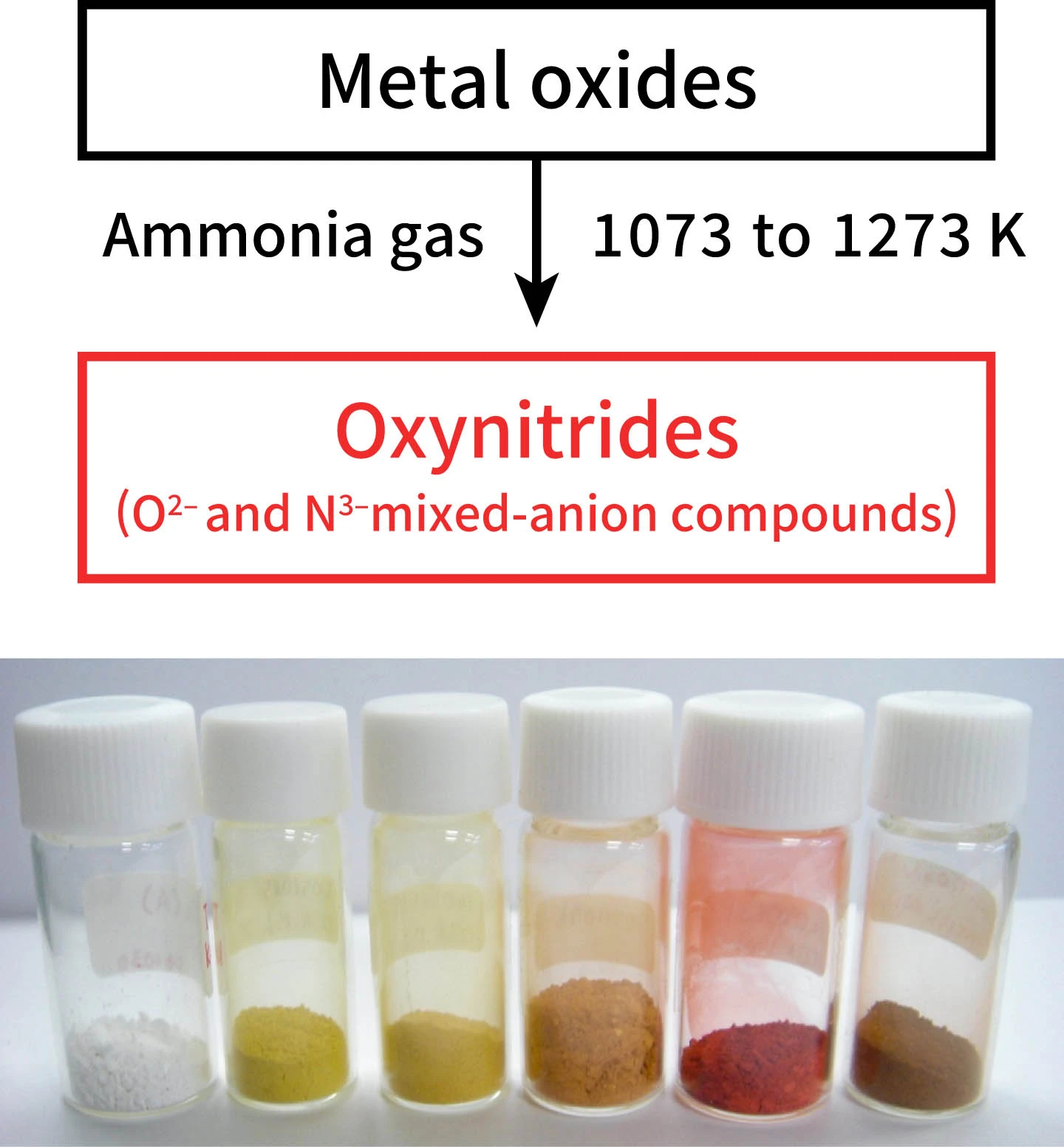
Figure 2: Oxynitride photocatalyst that absorbs visible light
Exposing metal oxides of different metals to high-temperature steam from ammonia gas allows oxynitrides of different colors to be synthesized.
At the same time, there are still major roadblocks to the practical use of mixed-anion compounds. One challenge is that the synthesis itself requires a large amount of energy. Also, it must be of high quality in order for it to work as a photocatalyst. “While the barriers to practical use are high, this just motivates me to work harder alongside the enthusiastic students in my laboratory, all driven by a desire to overcome challenges.“
Researching beyond conventional thinking and preconceptions
For the past decade, Maeda has been advancing research on photocatalysts, which are crucial for reducing carbon dioxide. “One of my collaborators suggested the possibility of using carbon nitride, which is an organic compound, as a photocatalyst, which got me started researching photocatalysts using organic compounds. At the time, inorganic compounds were used as photocatalysts, and no one was researching the use of organic compounds. However, carbon nitride absorbs visible light and is stable at high temperatures as well as in acids and bases. So, we tried using it and were amazed to find it functioned as a photocatalyst. By combining the carbon nitride with molecules that have catalytic properties, it was possible to apply it to carbon dioxide reduction (Figure 3).“

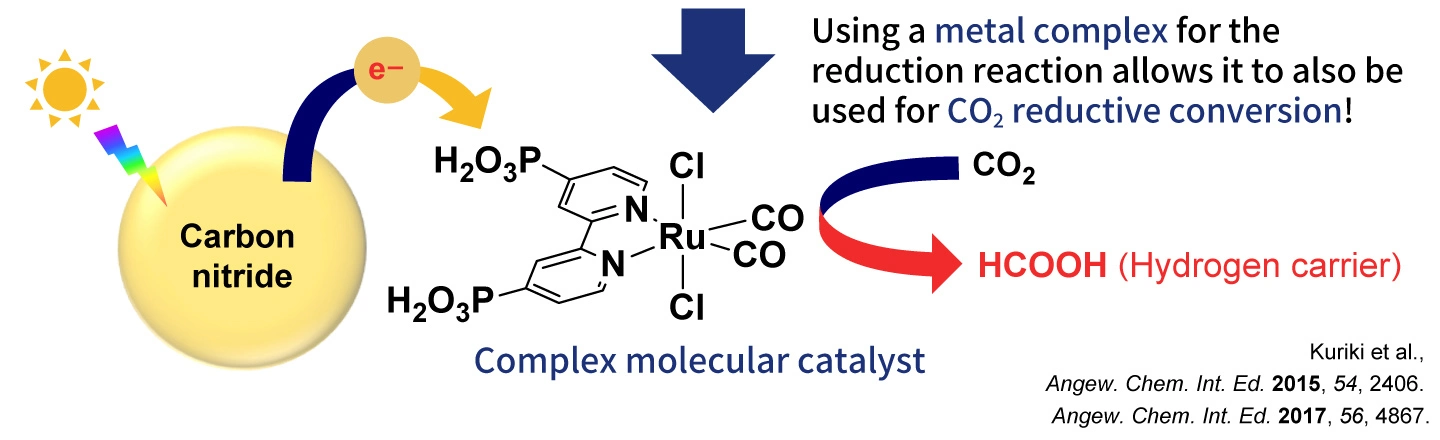
Figure 3: Development of a photocatalyst using organic carbon nitride and its application in carbon dioxide reduction
Since then, Maeda's research approach has been to try any substance with potential, without being constrained by conventional thinking or preconceptions. This has led to the discovery of various photocatalysts. Starting in 2018, he was selected by Clarivate as a Highly Cited Researcher in the field of Chemistry for four consecutive years, and has drawn attention from around the world.
Artificial photosynthesis—essential technology for a carbon neutral society
Maeda explained his strengths in the following way. “I believe it lies in my extensive collaboration with researchers both in Japan and internationally. I’ve been blessed with opportunities to participate in large-scale projects, which have connected me with many people. I have been able to work on experiments with them that couldn't be completed in my laboratory, and I was exposed to new ideas, which led me to unexpected achievements.“
Regarding his goals for the future, he added, “I want to send as many excellent students as possible who are willing to accept challenges out into society. That is my mission as a university professor.“

Meanwhile, regarding the practical application of artificial photosynthesis, he states, "Research on artificial photosynthesis is extremely challenging, and there are still many issues to address for its practical application. However, in recent years, a collaborative style among researchers, both domestically and internationally, has become established, so I believe that research and development toward practical application is definitely progressing as a result of this synergy. Solar power generation, which is another low-carbon technology, is also amazing, but it's nearly impossible to store electricity on a large scale by itself. Artificial photosynthesis is an essential technology for achieving a carbon neutral society because it is capable of splitting water to generate hydrogen, which is a key solution for this goal, on demand and producing chemical fuels such as hydrocarbons without relying on fossil fuels. I want to contribute to the field of artificial photosynthesis in terms of both research and human resource development."
Profile
Kazuhiko Maeda
Professor, Department of Chemistry, School of Science

- October 2024 to Present
- Professor, Department of Chemistry, School of Science, Institute of Science Tokyo
- 2022
- Professor, Department of Chemistry, School of Science, Tokyo Institute of Technology
- 2016
- Associate Professor, Department of Chemistry, School of Science, Tokyo Institute of Technology
- 2012
- Associate Professor, Department of Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology
- 2009
- Assistant Professor, School of Engineering, The University of Tokyo
- 2008
- Recipient of the JSPS Research Fellowship for Young Scientists (PD) at the Department of Chemistry, Pennsylvania State University
- 2007
- Recipient of the JSPS Research Fellowship for Young Scientists (PD) at the School of Engineering, The University of Tokyo
- 2006
- Recipient of the JSPS Research Fellowship for Young Scientists (DC2) at the School of Engineering, The University of Tokyo
- 2005
- Completed the master's program in the Department of Electronic Chemistry, Interdisciplinary Graduate School of Science and Engineering, Tokyo Institute of Technology
- 2003
- Graduated from the Department of Applied Chemistry, Faculty of Science Division 1, Tokyo University of Science
Interview Date: September 10, 2024 / 7th floor of the Environmental Energy Innovation Building (EEI Building), Ookayama Campus
Explore more details
Related articles
Science Tokyo Faces
“Faces“ is an article series introducing Science Tokyo researchers and their groundbreaking work. The series highlights their efforts to unravel the mysteries of our world and address the challenges facing our society.