Hidden architecture: identifying a new molecular structure in the center of chromosomes
Researchers discover a specialized histone arrangement that likely helps chromosomes separate correctly during cell division
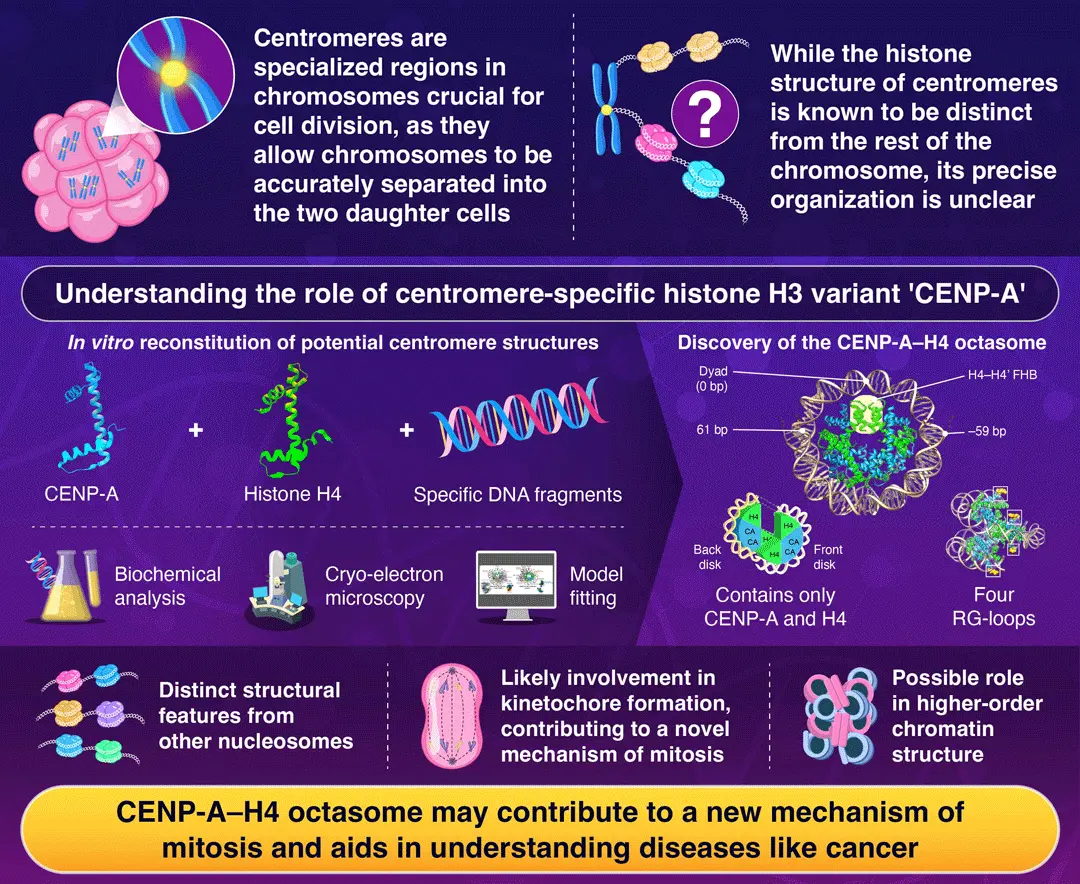
A newly identified centromeric structure, the CENP-A–H4 octasome, may reveal how chromosomes segregate properly during cell division, report researchers from Japan. Using cryo-electron microscopy, they visualized this complex formed only of histones CENP-A and H4, wrapped around 120 base pairs of DNA, revealing unique features important for mitosis. These findings may provide a novel perspective for understanding diseases caused by abnormal cell division, including cancer.
Unveiling a Novel Centromere-Specific Histone Variant in Chromosomes
Kawasaki et al. (2025) | Genes to Cells | 10.1111/gtc.70016
Centromeres, specialized regions located at the center of chromosomes, are critical for ensuring proper cell division. During mitosis, centromeres serve as assembly sites for complex protein structures called kinetochores, which connect chromosomes to the spindle apparatus to appropriately distribute genetic material to both daughter cells. Despite their fundamental biological role, the precise molecular architecture of centromeres has remained unclear, with scientists seeking to understand how these regions differ structurally from the rest of the chromosome.
In most chromosomal regions, DNA is wrapped around protein complexes called nucleosomes, which contain eight histone proteins—two each of H2A, H2B, H3, and H4. However, centromeres feature a specialized variant of histone H3, known as CENP-A, which marks these regions for kinetochore assembly. The exact composition of centromeric nucleosomes and how it contributes to the role of centromeres represents a significant knowledge gap in molecular biology.
Against this backdrop, a research team led by Associate Professor Kayo Nozawa from Institute of Science Tokyo, Japan, has recently made an important discovery on the detailed structure of centromeres. In their study, published in Volume 30, Issue 2 of Genes to Cells on March 24, 2025, the researchers elucidated the structure of a novel nucleosome-like particle, which is potentially localized to centromeric regions. The team reconstituted this complex using only CENP-A, histone H4 proteins, and DNA fragments; they intentionally excluded histones H2A and H2B, as these were known to be reduced in yeast centromeres.
Using cryo-electron microscopy, which enables visualization of frozen molecular structures at high resolution, the team analyzed this unique complex at a resolution of 3.66 Å (less than a billionth of a meter). The observed structure, which they named the ‘CENP-A–H4 octasome,’ exhibited characteristics quite distinct from conventional nucleosomes. “The CENP-A–H4 octasome has a structure where approximately 120 base pairs of DNA are wrapped around it, instead of the usual 145 base pairs. It also possesses four RG-loops derived from CENP-A that serve as a scaffold for proteins involved in kinetochore formation, potentially contributing to a new mechanism of mitosis,” explains Nozawa.
By consisting of only CENP-A and H4, this novel octameric arrangement has four exposed RG-loops, twice the number found in conventional CENP-A nucleosomes containing H2A and H2B. This likely supports binding to kinetochore-forming proteins. Additionally, the CENP-A-H4 octasome lacks the negatively charged surface known as the acidic patch, which typically serves as a binding platform for histone methyltransferases and chromatin remodelers that modulate higher-order chromatin structure. These findings suggest that significant differences in epigenetic regulation may exist between CENP-A–H4 octasomes and canonical nucleosomes.
The discovery of this unique centromeric structure provides valuable insights into how chromosomes segregate during cell division. Worth noting, errors in this process can lead to chromosomal abnormalities associated with various disorders. Overexpression or depletion of CENP-A has been reported to cause defects in chromosome segregation during cell division, and elevated levels of CENP-A expression have been observed in various cancers. “Our findings may provide new insights into understanding diseases caused by abnormal cell division, including cancer,” says Nozawa, with an eye on the future.
Overall, this research has advanced our understanding of chromosome organization and the specialized architecture needed for proper cell division.
Reference
- Authors:
- Osamu Kawasaki1, Yoshimasa Takizawa2,3, Iori Kiyokawa1, Hitoshi Kurumizaka2,4,5*, and Kayo Nozawa1*
- Title:
- Cryo-EM Analysis of a Unique Subnucleosome Containing Centromere-Specific Histone Variant CENP-A
- Journal:
- Genes to Cells
- DOI:
- 10.1111/gtc.70016
- Affiliations:
- 1School of Life Science and Technology, Institute of Science Tokyo, Japan
2Laboratory of Chromatin Structure and Function, Institute for Quantitative Biosciences, The University of Tokyo, Japan
3Department of Computational Biology and Medical Sciences, Graduate School of Frontier Sciences, The University of Tokyo, Japan
4Department of Biological Sciences, Graduate School of Science, The University of Tokyo, Japan
5Laboratory for Transcription Structural Biology, RIKEN Center for Biosystems Dynamics Research, Japan
Related articles
Further Information
Associate Professor Kayo Nozawa
School of Life Science and Technology, Institute of Science Tokyo
Contact
Public Relations Division, Institute of Science Tokyo
- Tel
- +81-3-5734-2975
- media@adm.isct.ac.jp