AI-guided protein design for enhanced intracellular antibodies
Researchers use a machine learning approach to improve the stability and performance of antibodies inside cells
A new artificial intelligence-driven pipeline developed in a collaborative research combines protein structure prediction, sequence design, and live-cell screening together to enable rapid conversion of antibody sequences into functional intracellular antibodies (intrabodies) that are stable within living cells. By preserving antigen-binding regions and improving structural stability, the approach overcomes major barriers encountered in intrabody development—emerging as a simpler, more cost-effective tool for diagnostics, imaging, and biomedical research.
AI-Driven Protein Design: Making Intrabodies Work Inside Cells
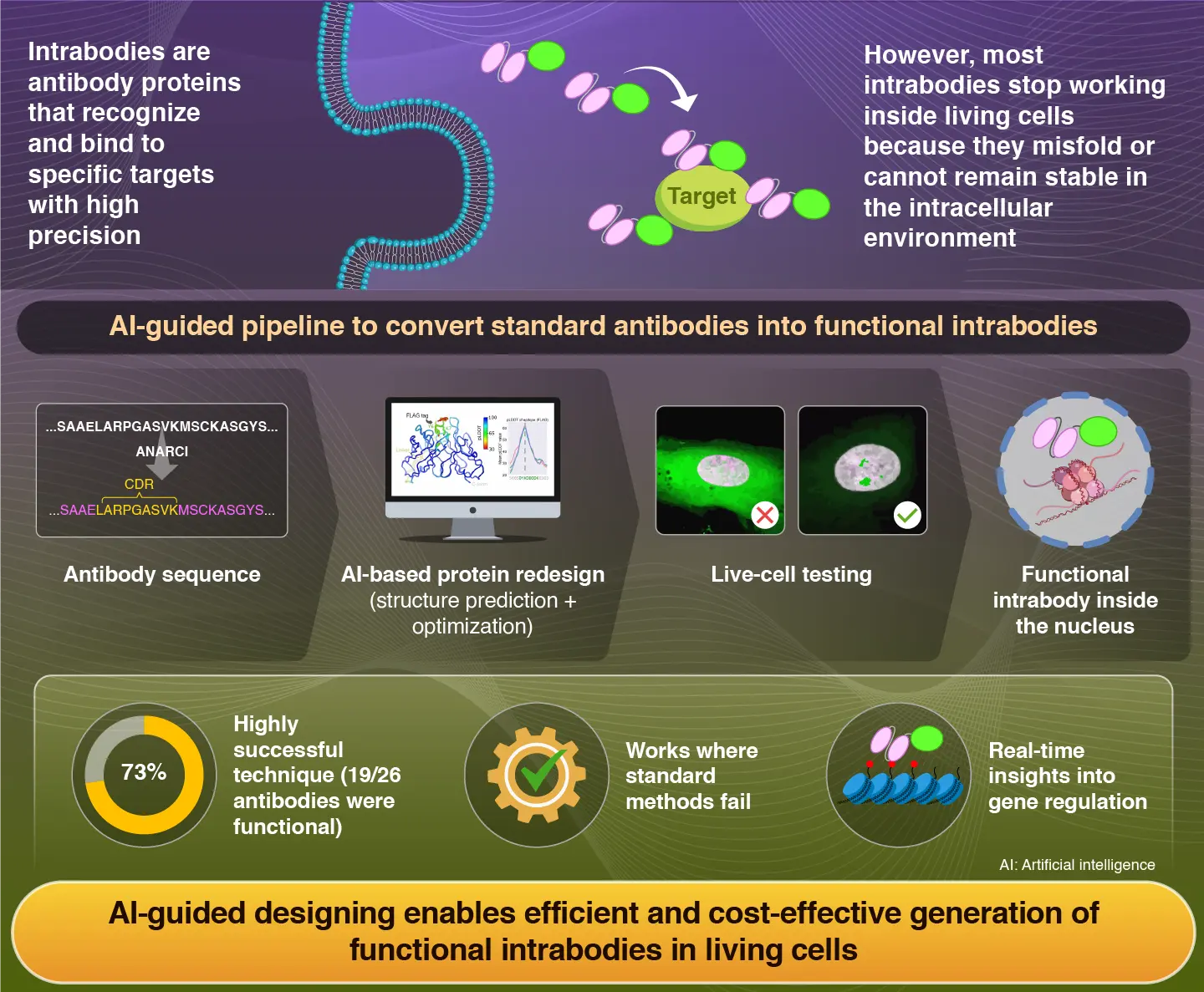
Antibodies are proteins that are widely used in biology and medicine. These are target-selective and can precisely recognize and bind to their targets. Due to their selectivity, they are often used to detect and study specific molecules. However, most conventional antibodies only work outside living cells, which limits their ability to probe important biological processes that occur inside cells. Intracellular antibodies (also known as intrabodies) offer a way to overcome this limitation by functioning within living cells. However, developing intrabodies has proven to be quite challenging as antibodies tend to misfold or lose activity within the cells.
To tackle this issue, a team of researchers led by Professor Hiroshi Kimura from the Institute of Integrated Research, Institute of Science Tokyo (Science Tokyo), Japan, along with Mr. Daiki Maejima, a third-year doctoral student from Science Tokyo, Associate Professor Timothy J. Stasevich of Colorado State University, USA, and Professor Yasuyuki Ohkawa of Kyushu University, Japan, developed a new design strategy that uses artificial intelligence to design functional intrabodies. Their findings were published in Volume 12, Issue 1 of the journal Science Advances on January 02, 2026.
"We created a pipeline that combines artificial intelligence (AI)-guided protein structure prediction with sequence redesign and live-cell screening," says Kimura.
The method keeps the target-binding regions intact and redesigns the surrounding framework regions. This ensures that the antibodies fold appropriately and stay stable within the cells without compromising their ability to bind to specific molecules.
The team tested around 26 existing antibody sequences. Among these, 19 were successfully converted into functional intrabodies. Interestingly, 18 of these had previously failed to work as functional intrabodies when researchers used conventional approaches.
"Many antibodies that were not functional when expressed as intrabodies were ultimately found to be converted into functional ones in our study," comments Kimura. "This confirms that AI allowed us to redesign structures that were compatible within the cellular environment."
The major focus of the study was intrabodies that specifically target modifications in histone proteins (proteins that play a key role in packaging DNA and regulating gene activity). These modifications can serve as stable markers of gene activity or can change rapidly and are difficult to study using traditional labeling techniques. The newly designed intrabodies were able to detect these changes within living cells and responded as modification levels increased or decreased based on fluorescence, demonstrating high potential for studying gene regulation.
Further experiments confirmed that the redesigned molecules were stable, functional, soluble, and retained high target specificity within the living cell. Moreover, they also showed consistent and predictable behavior across varying cellular conditions, highlighting the reliability of the research tool.
"By combining AI-based design with live-cell testing, we can now accelerate intrabody development with far greater confidence," explains Kimura.
Beyond basic research, this approach has the potential to streamline intrabody development, making it faster, cheaper, and more accessible. As antibody sequence databases continue to expand, the ability to convert existing antibodies into functional intracellular probes could enable a broad range of applications, from diagnostics and fluorescence imaging to the development of therapeutic strategies. Overall, the study shows how AI can help unlock the full potential of antibodies, opening new paths for exploring complex biological processes.
Reference
- Authors:
- Gabriel Galindo1, Daiki Maejima2, Jacob DeRoo3, Scott R. Burlingham1, Gretchen Fixen1,
Tatsuya Morisaki1, Hallie P. Febvre1, Ryan Hasbrook1, Ning Zhao4, Soham Ghosh3,5,
E. Handly Mayton6, Christopher D. Snow1,3,7, Brian J. Geiss3,6, Yasuyuki Ohkawa8, Yuko Sato8,9, Hiroshi Kimura2,9,10, Timothy J. Stasevich1,3,7,10 - Title:
- AI-assisted protein design to rapidly convert antibody sequences to intrabodies targeting diverse peptides and histone modifications
- Journal:
- Science Advances
- Affiliations:
- 1Department of Biochemistry and Molecular Biology, Colorado State University, USA.
2School of Life Science and Technology, Institute of Science Tokyo, Yokohama, Japan.
3School of Biomedical Engineering, Colorado State University, USA.
4Department of Biochemistry and Molecular Genetics, University of Colorado-Anschutz Medical Campus, USA.
5Department of Mechanical Engineering, Colorado State University, USA.
6Department of Microbiology, Immunology, & Pathology, Colorado State University, USA.
7Department of Chemical & Biological Engineering, Colorado State University, USA.
8Medical Institute of Bioregulation, Kyushu University, Japan.
9Cell Biology Center, Institute of Innovative Research, Tokyo Institute of Technology, Japan.
10Cell Biology Center, Institute of Integrated Research, Institute of Science Tokyo, Japan.
Related articles
Further information
Professor Hiroshi Kimura
School of Life Science and Technology, Institute of Science Tokyo
Contact
Public Relations Division, Institute of Science Tokyo
- media@adm.isct.ac.jp
- Tel
- +81-3-5734-2975