A Sustainable Iron Catalyst for Water Oxidation in Renewable Energy
A breakthrough iron-based catalyst achieves near-perfect efficiency for water oxidation, offering a sustainable solution for hydrogen production
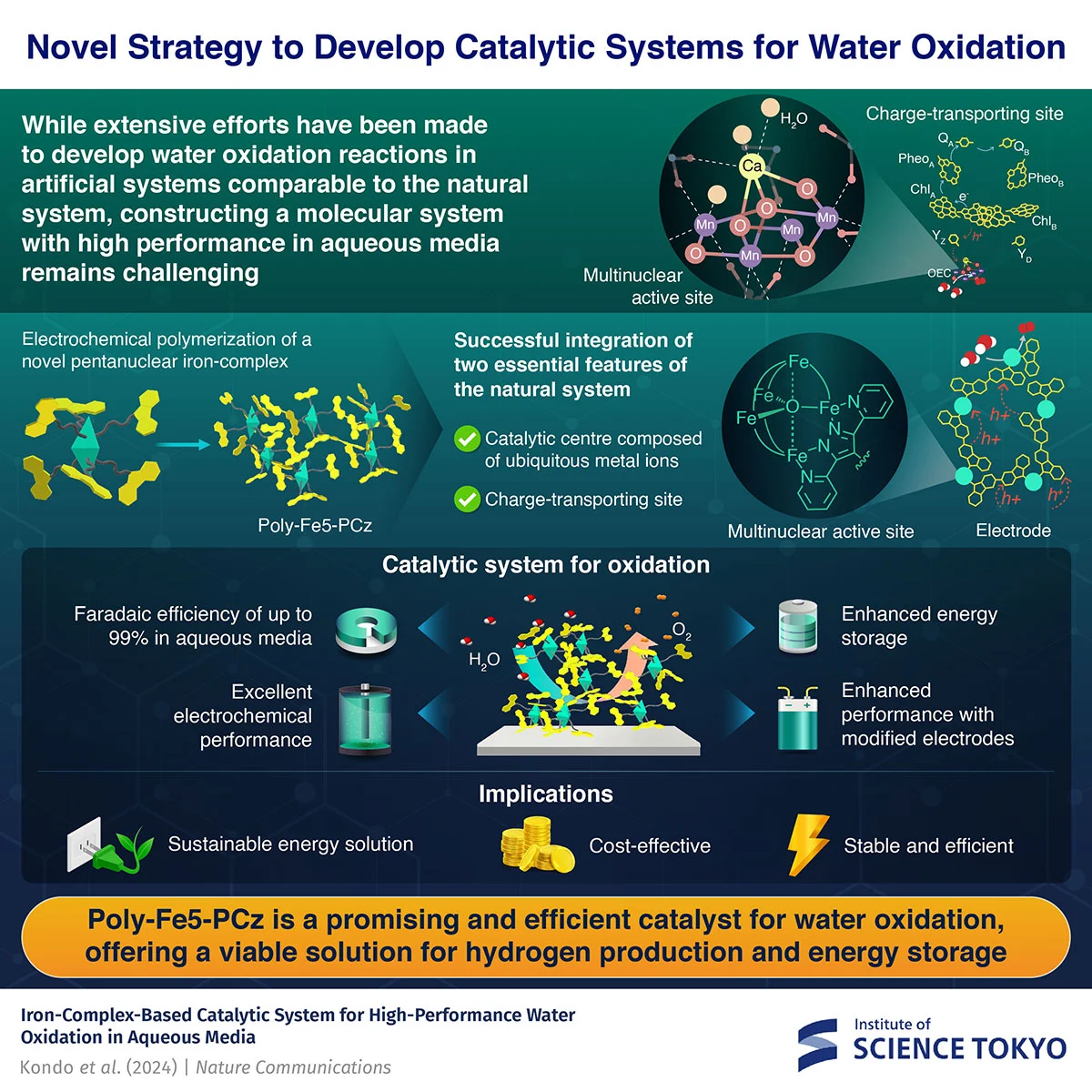
A newly developed pentanuclear iron complex (Fe5-PCz (ClO4)3) can offer an efficient, stable, and cost-effective solution for water oxidation. By electrochemically polymerizing the complex, researchers from Institute of Science Tokyo obtained a polymer-based catalyst, poly-Fe5-PCz, and achieved water oxidation with up to 99% Faradaic efficiency and exceptional stability, even under rigorous conditions. This breakthrough offers a scalable alternative to rare metal catalysts, advancing hydrogen production and energy storage for renewable energy.
Water oxidation plays a vital role in renewable energy technologies, especially in hydrogen production and artificial photosynthesis. By splitting water into oxygen and hydrogen, it provides a clean, sustainable energy source. However, replicating the efficiency and stability of natural photosynthetic systems in artificial catalytic setups—especially in aqueous environments—remains a significant challenge. Catalysts based on rare and expensive metals like ruthenium have shown high activity for water oxidation but are not practical for large-scale use due to their cost and limited availability.
To address this, a team of researchers led by Professor Mio Kondo from Institute of Science Tokyo (Science Tokyo), Japan, developed a more sustainable and cost-effective catalytic system using abundant metals. Their findings were published in Nature Communications on March 5.
The study introduces a novel pentanuclear iron complex, Fe5-PCz (ClO4)3, which possesses a multinuclear-complex-based catalytically active site and precursor moieties for charge transfer sites. Kondo explains, "By electrochemically polymerizing this multinuclear iron complex, we create a polymer-based material that enhances electrocatalytic activity and long-term stability. This approach combines the benefits of natural systems with the flexibility of artificial catalysts, paving the way for sustainable energy solutions."
The researchers synthesized the Fe5-PCz (ClO4)3 complex using organic reactions like bromination, nucleophilic substitution, Suzuki coupling reactions, and subsequent complexation reactions. The synthesized complex was characterized by mass spectrometry, elemental analysis, and single-crystal X-ray structural analysis. The researchers then modified glassy carbon and indium tin oxide electrodes by polymerizing Fe5-PCz using cyclic voltammetry and controlled potential electrolysis to afford a polymer-based catalyst, poly-Fe5-PCz. The charge transfer ability and electrocatalytic performance of poly-Fe5-PCz were evaluated through electrochemical impedance spectroscopy and oxygen evolution reaction (OER) experiments with oxygen production quantified by gas chromatography, respectively.
The results were highly promising. Kondo explains, “Poly-Fe5-PCz achieved up to 99% Faradaic efficiency in aqueous media, meaning nearly all the applied current contributed to the OER. The system also exhibited superior robustness and a reaction rate under rigorous testing conditions compared to relevant systems. Additionally, poly-Fe5-PCz demonstrated enhanced energy storage potential and improved electrode compatibility, making it suitable for a wide range of renewable energy applications.” Its high stability was further confirmed by long-term controlled potential experiments, a key advantage for hydrogen production and energy storage technologies.
The study's findings have significant implications for sustainable energy. The use of iron—an abundant, non-toxic metal—ensures the system is both eco-friendly and cost-effective, offering a viable alternative to precious metal-based catalysts. Its stability under operational conditions addresses a major challenge in artificial catalytic systems, where long-term catalyst degradation often limits performance. Moreover, the system's performance in aqueous environments makes it suitable for applications in water splitting.
“Optimizing poly-Fe5-PCz synthesis and scalability could further enhance its performance, paving the way for industrial-scale hydrogen production and energy storage. Our study opens new possibilities for integrating the system into broader energy technologies, paving the way to a more sustainable future,” concludes Kondo.
Reference
- Authors:
- Takumi Matsuzaki1, Kento Kosugi1,2, Hikaru Iwami1, Tetsuya Kambe1,3, Hisao Kiuchi4, Yoshihisa Harada4, Daisuke Asakura5, Taro Uematsu1,3, Susumu Kuwabata1, Yutaka Saga1,3, Mio Kondo1,2,6*, Shigeyuki Masaoka1,3*
*Corresponding authors - Title:
- Iron-Complex-Based Catalytic System for High-Performance Water Oxidation in Aqueous Media
- Journal:
- Nature Communications
- Affiliations:
- 1 Division of Applied Chemistry, Osaka University, Japan
2 Department of Chemistry, Institute of Science Tokyo, Japan
3 Innovative Catalysis Science Division, Osaka University, Japan
4 Institute for Solid State Physics, The University of Tokyo, Japan
5 Research Institute for Energy Conservation, National Institute of Advanced Industrial Science and Technology (AIST), Japan
6 JST, PRESTO, Japan
Related articles
Further Information
Professor Mio Kondo
Department of Chemistry, School of Science, Institute of Science Tokyo
- mio@chem.sci.isct.ac.jp
Contact
Public Relations Division, Institute of Science Tokyo
- Tel
- +81-3-5734-2975
- media@adm.isct.ac.jp