Discovering the Role of MicroRNA-27a in Tissue Regeneration and Bone Healing
Scientists explore the role of microRNA-27a in tissue regeneration in human dental pulp stem cells
MicroRNA-27a has been shown to stimulate both the wingless-type integration site family, or Wnt signaling, and the bone morphogenetic protein pathways to actively promote bone regeneration, according to a recent study from Institute of Science Tokyo, Japan. Their important findings shed light on the intrinsic cellular pathways and mechanisms that are critical for the development of new bone-like tissue and could inform the design and development of future tissue regeneration therapies.
Unveiling the Role of MicroRNA-27a in Dental Tissue Regeneration
Yu et al. (2025) | Journal of Translational Medicine | 10.1186/s12967-025-06208-9
Dental caries, or tooth decay, is a common oral health condition that often causes significant pain and discomfort and may even lead to tooth loss. In severe and untreated cases, bacterial infection combined with the host’s immune response can cause bone resorption, or the breakdown of bone tissue in the tooth root. Moreover, traditional treatments for advanced dental caries, such as surgery, can result in bone defects that require complex bone grafting procedures.
Building on this knowledge, bone tissue engineering and dental tissue regeneration have gained the attention of researchers worldwide. Recent reports suggest that microRNAs (miRNAs)—small, non-coding ribonucleic acid sequences—play a key role in bone tissue regeneration. However, the underlying mechanisms and pathways regulated by miRNAs remain unclear.
To investigate the intrinsic processes involved in dental bone repair, a team of researchers led by Associate Professor Nobuyuki Kawashima, graduate student Ziniu Yu, and Professor Takashi Okiji from the Graduate School of Medical and Dental Sciences, Institute of Science Tokyo (Science Tokyo), Japan, conducted a series of innovative experiments using human dental pulp stem cells (hDPSCs) and mice. Their findings were published in Volume 23 and Issue 189 of the Journal of Translational Medicine on February 16, 2025.
“hDPSCs are a type of mesenchymal stem cell that have the ability to differentiate into either odontoblasts or osteoblasts, key players in dental tissue repair,” explains Kawashima. “In our study, we focused on a molecule called miRNA-27a, which we found to exert an anti-inflammatory effect by suppressing the NF-κB pathway but may also promote tissue regeneration by activating Wnt and BMP signaling. By overexpressing miRNA-27a in hDPSCs, we explored how it might guide these cells toward becoming hard tissue-forming cells.”
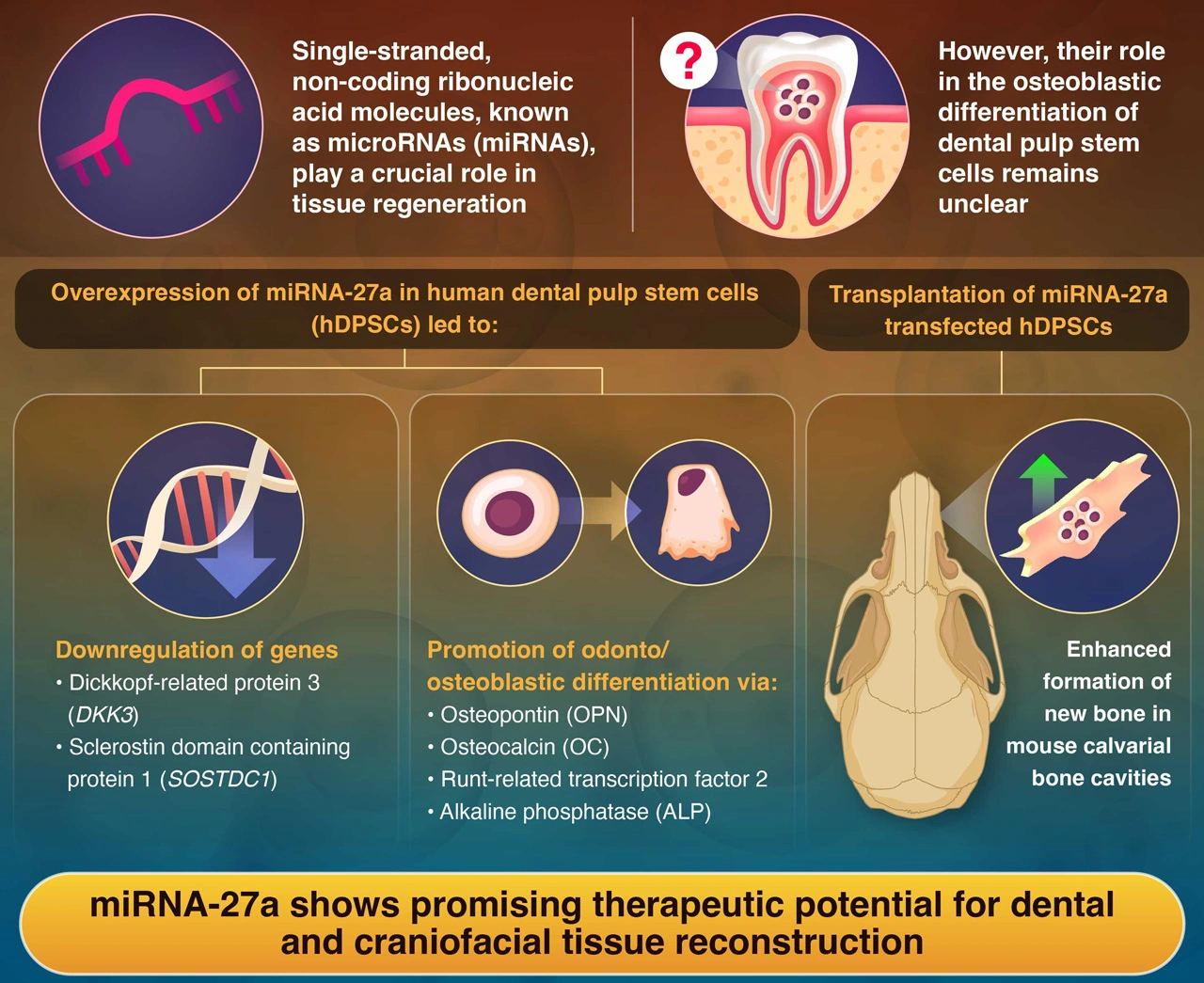
Initially, the scientists observed that miRNA-27a was markedly elevated in hDPSCs committed to odonto-/osteoblastic differentiation. Additionally, the expression of odonto-/osteoblastic markers and mineralized nodule formation were significantly upregulated in hDPSCs transfected with the miRNA-27a mimic. Furthermore, the miRNA-27a mimic increased the mRNA expression of transcription factor 7 (TCF7) and lymphoid enhancer-binding factor 1 (LEF1), which are essential transcription factors in the Wnt signaling pathway, in hDPSCs. The researchers identified dickkopf-related protein 3 (DKK3) and sclerostin domain-containing protein 1 (SOSTDC1), which are considered potential negative regulators of the Wnt signaling pathway, as the primary target genes regulated by miRNA-27a. This suggests that miRNA-27a helps remove these biological brakes, enabling the cells to activate bone-forming signals more efficiently.
In addition to stimulating the Wnt pathway, miRNA‑27a was found to significantly influence the odonto/osteoblastic differentiation of hDPSCs and activate the bone morphogenetic protein (BMP) pathway. Activation of both the Wnt and BMP pathways suggested that hard-tissue-forming cells were promoted through differentiation of hDPSCs.
To validate their findings, the researchers transplanted collagen scaffolds containing miRNA-27a-expressing hDPSCs into the artificial defects introduced on the calvarial bone of mice. Subsequent analyses revealed the formation of new bone-like tissue, which was absent in the control group.
Kawashima concludes by highlighting the therapeutic potential of the research: “These results suggest that miRNA-27a could play a pivotal role in encouraging bone-like tissue formation. This opens up exciting possibilities for advancing regenerative therapies aimed at repairing dental and craniofacial defects.”
In summary, this study underscores the significant translational potential of miRNA-27a in promoting dental tissue regeneration.
Reference
- Authors:
- Ziniu Yu1, Nobuyuki Kawashima1*, Keisuke Sunada‑Nara1, Shihan Wang1, Peifeng Han1,
Thoai Quoc Kieu2, Chunmei Ren1, Sonoko Noda1, Kento Tazawa1, and Takashi Okiji1 - Title:
- MicroRNA-27a transfected dental pulp stem cells undergo odonto/osteogenic differentiation via targeting DKK3 and SOSTDC1 in Wnt/BMP signaling in vitro and enhance bone formation in vivo
- Journal:
- Journal of Translational Medicine
- Affiliations:
- 1Graduate School of Medical and Dental Sciences, Institute of Science Tokyo, Japan
2Department of Pediatric Dentistry, University of Medicine and Pharmacy, Vietnam
Related articles
Further Information
Associate Professor Nobuyuki Kawashima
Department of Pulp Biology and Endodontics, Graduate School of Medical and Dental Sciences, Institute of Science Tokyo
Contact
Public Relations Division, Institute of Science Tokyo
- media@adm.isct.ac.jp
- Tel
- +81-3-5734-2975