Novel Catalyst Development for Sustainable Ammonia Synthesis
This study unveils Ba-Si orthosilicate oxynitride-hydride as a transition metal-free catalyst, paving the way for sustainable chemical innovation
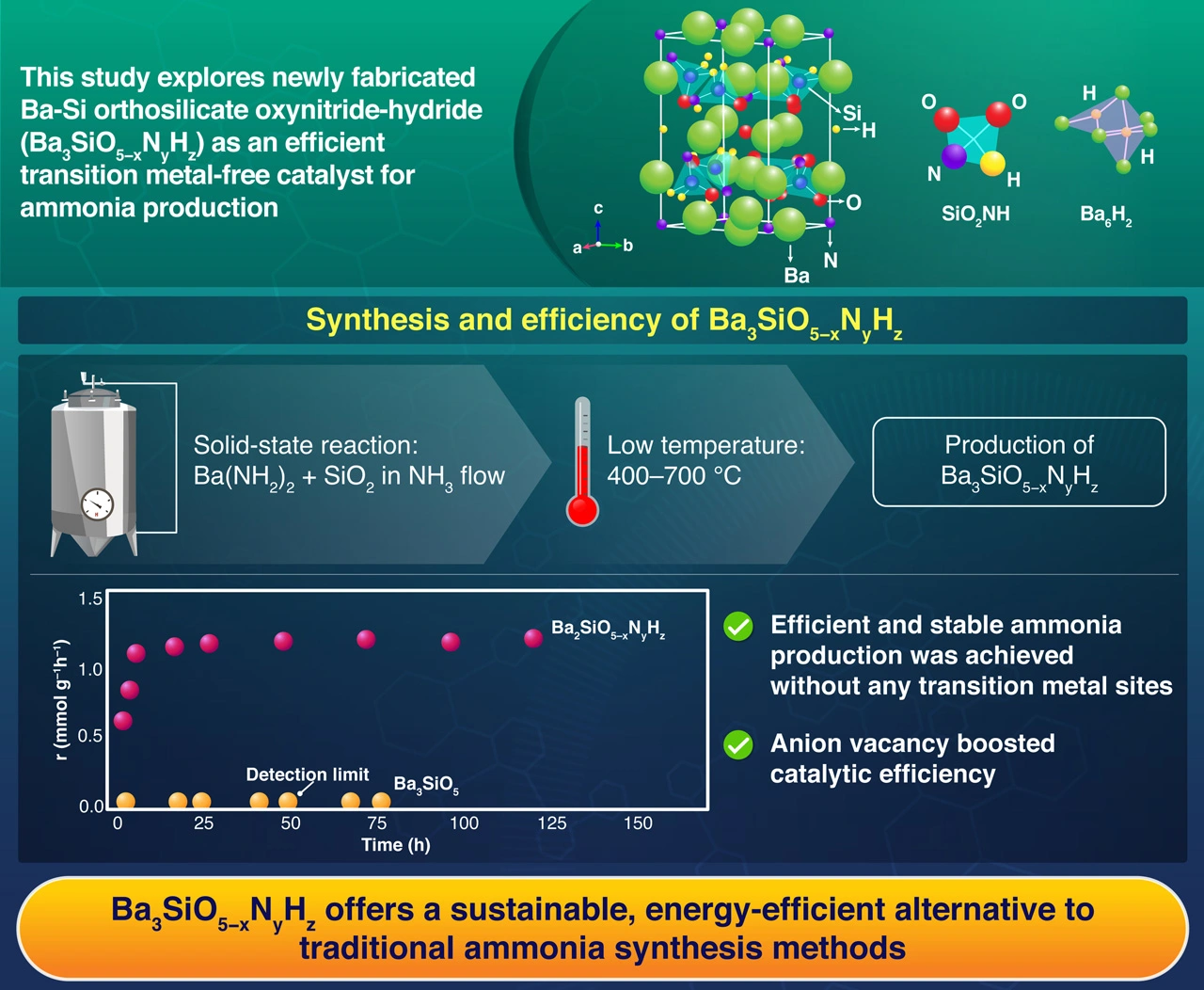
A groundbreaking study explores Ba-Si orthosilicate oxynitride-hydride (Ba3SiO5−xNyHz) as a sustainable catalyst for ammonia synthesis, offering a potential alternative to traditional transition metal-based systems. Synthesized through low-temperature solid-state reactions and enhanced with ruthenium nanoparticles, these compounds demonstrated improved catalytic performance under milder conditions, providing a more energy-efficient route to ammonia production. This approach also addresses the environmental challenges associated with conventional methods, signaling a shift toward greener industrial practices in ammonia production.
Efficient Ammonia Production via Anion Vacancy on Silicon-based Oxide
Zhang et al. (2025) | Nature Chemistry | 10.1038/s41557-025-01737-8
As the world moves toward sustainability, the demand for efficient alternatives across industries continues to grow. Ammonia, a key chemical used in fertilizers, explosives, and various other products, is primarily synthesized through the energy-intensive Haber-Bosch process. This process requires extremely high temperatures and pressures, contributing to global carbon dioxide emissions. Conventional catalysts, such as iron and ruthenium, rely on these harsh conditions to drive the reaction. However, a recent study by researchers from Institute of Science Tokyo, the National Institute for Materials Science, and Tohoku University, Japan, led by Professor Masaaki Kitano, explores Ba3SiO5−xNyHz catalyst as a sustainable alternative to traditional catalysts, potentially revolutionizing ammonia synthesis.
Vacancies, especially anion vacancies within the three-dimensional structure of catalysts, function as active sites. These active sites are energetically involved in the process of catalysis. However, anion vacancies alone are not effective without the presence of transition metal sites. This limitation inspired researchers to develop a transition metal-free catalyst.
Published online in Nature Chemistry on 17 February 2025, the study aims to develop more efficient, sustainable ammonia synthesis methods. Kitano explains, "We have focused on tribarium silicate (Ba3SiO5) for the synthesis of our novel catalyst due to its unique crystal structure and chemical properties, offering the potential to lower energy requirements and reduce operating conditions," describing the initial step in their research. To address the environmental and energy challenges posed by conventional synthesis methods, the research team developed and tested various mixed-anion materials.
The study progressed through several stages. First, the researchers synthesized a novel Ba-Si oxynitride-hydride, Ba3SiO5−xNyHz, through a low-temperature (400–700 °C) solid-state reaction of barium amide with silicon dioxide. The resulting chemical composition was determined to be Ba3SiO2.87N0.80H1.86. This synthesis temperature is much lower than the synthesis temperatures (1100–1400 °C) of conventional silicate materials such as Ba3SiO5, Ba3Si6O9N4, and BaSi2O2N2. The synthesized Ba3SiO5−xNyHz demonstrated exceptional stability as a catalyst for ammonia synthesis even in the absence of any transition metal sites. It showed higher activity and lower activation energy than the conventional ruthenium-loaded MgO catalyst. On the other hand, Ba3SiO5, Ba3Si6O9N4, and BaSi2O2N2 exhibited no catalytic activity. The Ba3SiO5−xNyHz catalyst’s ammonia synthesis activity was tested under varying temperatures and pressures, and structural properties were analyzed using advanced instrumentation techniques.
To further improve performance, ruthenium nanoparticles were introduced. The researchers found that Ba3SiO5−xNyHz showed the highest catalytic activity with ruthenium nanoparticles. "The addition of ruthenium nanoparticles significantly boosted catalytic performance, enabling more efficient ammonia synthesis under milder conditions. However, the main active site is not ruthenium nanoparticles but the anion vacancy sites on Ba3SiO5−xNyHz, which reduces the apparent energy requirement for ammonia synthesis than conventional catalysts. We also discovered that the anion vacancy-mediated mechanism played a key role in facilitating nitrogen activation, without relying on transition metals," says Kitano. These findings suggest a more sustainable and energy-efficient path for ammonia synthesis.
The study highlights key benefits: reduced temperature and pressure enhance efficiency, while the transition metal-free pathway cuts emissions and resource dependence, supporting sustainability. Furthermore, the scalable synthesis and robust performance of Ba3SiO5−xNyHz catalysts position them as promising candidates for industrial adoption, offering a more sustainable approach to ammonia production at scale. These findings also open avenues for further research in transition metal-free catalysis for other critical processes.
This study represents a significant step toward sustainable ammonia synthesis, addressing a major challenge in industrial chemistry. By demonstrating the potential of the Ba3SiO5−xNyHz catalyst, the researchers have laid the foundation for a greener and more efficient approach to producing ammonia, an essential chemical. As the global demand for ammonia continues to rise, innovations like this will be instrumental in driving a more sustainable future.
Reference
- Authors:
- Zhujun Zhang1, Kazuki Miyashita1, Tong Wu1, Jun Kujirai1, Kiya Ogasawara1, Jiang Li1, Yihao Jiang1, Masayoshi Miyazaki1, Satoru Matsuishi1,2, Masato Sasase1, Tomofumi Tada1*, Hideo Hosono1,2*, Masaaki Kitano1,3*
*Corresponding authors - Title:
- Anion vacancies activate N2 to ammonia on Ba-Si orthosilicate oxynitride-hydride
- Journal:
- Nature Chemistry
- Affiliations:
- 1MDX Research Center for Element Strategy, Institute of Integrated Research, Institute of Science Tokyo, Japan
2MANA Center, National Institute for Materials Science, Japan
3Advanced Institute for Materials Research (WPI-AIMR), Tohoku University, Japan
Related articles
Further information
Professor Masaaki Kitano
Institute of Integrated Research, Institute of Science Tokyo
Contact
Public Relations Division, Institute of Science Tokyo
- Tel
- +81-3-5734-2975
- media@adm.isct.ac.jp