Cell Connects: Breaking Barriers in Stem Cell Communication Through mRNA Transfer
Study reveals a new mechanism by which cells naturally exchange genetic information, with significant biological effects
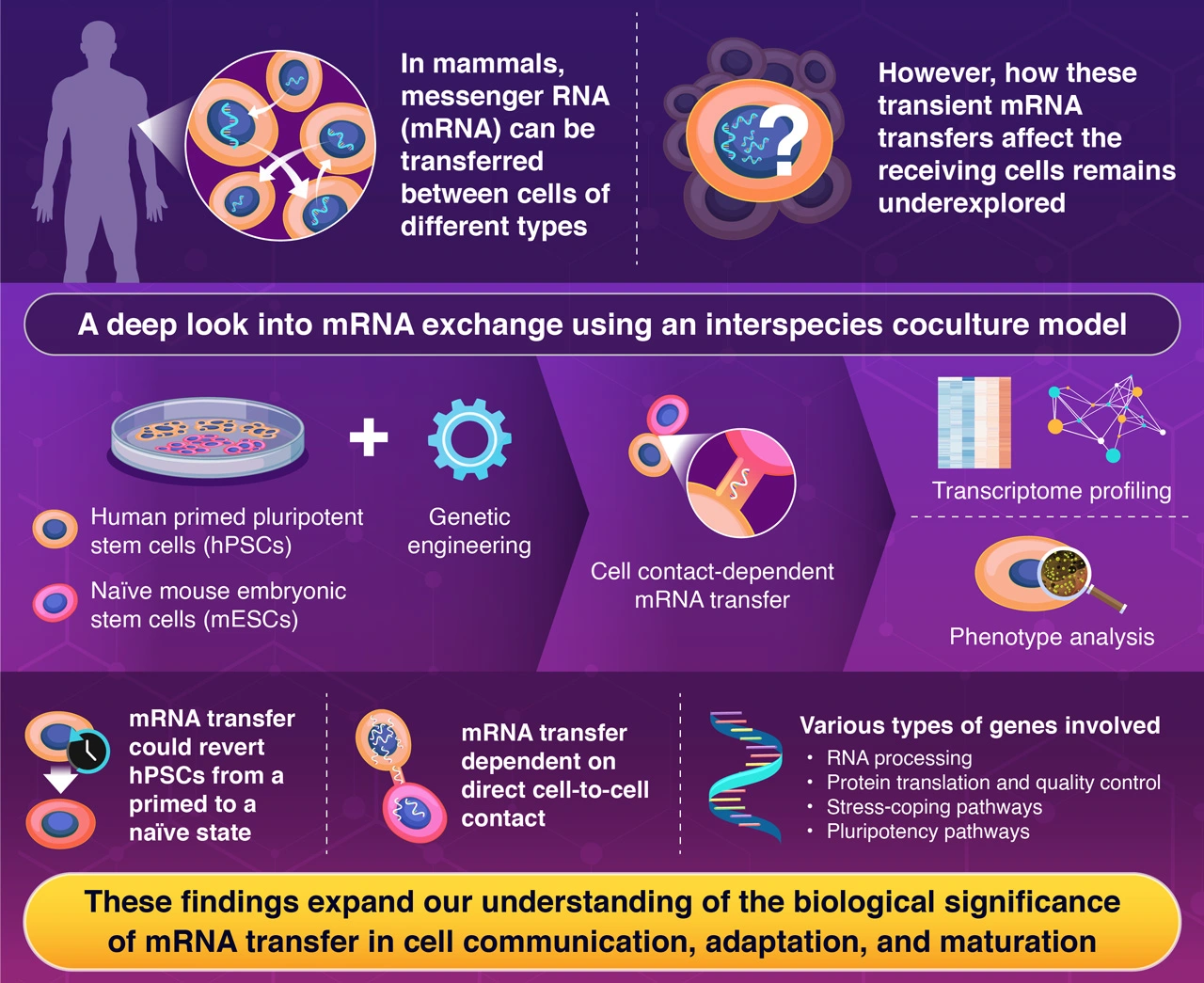
Messenger RNA can travel between different types of stem cells through tunnel-like structures, as revealed by a new study. By studying interactions between mouse and human stem cells, they discovered that this RNA transfer can reprogram human cells to an earlier developmental state. This groundbreaking finding not only sheds light on an underexplored form of cellular communication but also suggests promising applications in regenerative medicine without using artificial genetic modifications or external chemicals.
Shedding Light on Genetic Material Exchanges Between Mammalian Cells
Yoneyama et al. (2025) | Proceedings of the National Academy of Sciences |10.1073/pnas.2413351122
Cell-to-cell communication is essential throughout all forms and stages of life, and many communication mechanisms are well studied. However, over the past few years, scientists have found increasing evidence that RNA, which carries genetic information and regulates gene expression, is also involved in intercellular communication.
One way in which messenger RNA (mRNA) transmission happens is through extracellular vesicles. Cells secrete small-sized, membranous vesicles containing biomolecules, including RNA, and these are taken up by nearby cells. A less understood mechanism of mRNA transfer involves tubular structures that form when cells come into contact. However, very few reports on this type of mRNA movement have been made, and the biological significance of this type of communication in stem cells remains mostly a mystery.
Against this backdrop, a research team led by Professor Takanori Takebe from Institute of Science Tokyo, Japan, investigated the mechanisms and roles of mRNA transfer between different types of stem cells. Their findings were published in the journal Proceedings of the National Academy of Sciences on January 22, 2025, in Volume 122, Issue 4.
To more easily detect mRNA trafficking from one cell to another, the researchers employed a coculture experimental system. Simply put, they cultured mouse embryonic stem cells (mESCs) alongside human primed pluripotent stem cells (hPSCs). “When we started this coculture for a different purpose, we almost serendipitously found this unexpected mRNA transfer phenomenon as we could distinguish endogenously expressed genes and laterally transferred mRNAs based on genetic sequence differences between mice and humans,” explains Takebe.
Using this experimental system, coupled with RNA imaging analysis and mouse-specific gene expression analysis, the team revealed that mRNA from mESCs moved into hPSCs during co-culture. An in-depth analysis of this transferred mRNA revealed that mRNAs coding for molecules related to transcription, translation, and stress response were transferred from mouse cells to human cells. They also proved that this mRNA transfer occurred through tunnel-like membrane extension structures, called “tunneling nanotubes,” formed between mouse and human cells.
Next, the researchers investigated the biological effects that this transferred mRNA had on the receiving cells. Notably, they found that primed hPSCs could be reverted to a so-called “naïve” state. In other words, the human cells reverted to an earlier embryonic stage in their differentiation. This suggests that mRNA moving between different mammalian stem cells has biologically significant effects beyond mere movement, going as far as cell fate conversion. The team also identified several key transcription factors involved in this process, with essential functions for pluripotent state maintenance.
Taken together, the results of this study shed light on the importance of intercellular mRNA transfer. First author Yoneyama says,“This study provides insights into a novel mechanism of intercellular communication, illustrating how cell populations coordinate and coexist with their surrounding environment, thereby advancing our understanding of biological phenomena.” Takebe concludes, “We expect our findings to contribute to the development of new cell-fate control technology that does not rely on artificial gene introduction or chemical compounds.” Such technologies could lead to new therapeutic strategies in regenerative medicine and novel drugs.
Further efforts will be needed to fully grasp the intricacies of cell-to-cell communication, and Takebe’s group is already looking forward to pursuing this exciting line of research.
Reference
- Authors:
- Yosuke Yoneyama1,2, Ran-Ran Zhang3,4,5, Mari Maezawa1, Hideki Masaki6, Masaki Kimura3,4,5, Yuqi Cai3,4,5, Mike Adam3,4,5, Sreeja Parameswaran7, Naoaki Mizuno6, Joydeep Bhadury8, So Maezawa9, Hiroshi Ochiai10, Hiromitsu Nakauchi6,8, S. Steven Potter3,4,5, Matthew T. Weirauch3,4,5,7,11, and Takanori Takebe1,2,3,4,5,11,12,13
- Title:
- Intercellular mRNA transfer alters the human pluripotent stem cell state
- Journal:
- Proceedings of the National Academy of Sciences
- Affiliations:
- 1Human Biology Research Unit, Institute of Integrated Research, Institute of Science Tokyo, Japan
2Department of Genome Biology, Graduate School of Medicine, Osaka University, Japan
3Division of Gastroenterology, Hepatology & Nutrition, Cincinnati Children’s Hospital Medical Center, USA
4Division of Developmental Biology, Cincinnati Children’s Hospital Medical Center, USA
5Division of Biomedical Informatics, Cincinnati Children’s Hospital Medical Center, USA
6Stem Cell Therapy Division, Institute of Integrated Research, Institute of Science Tokyo, Japan
7Center for Autoimmune Genomics and Etiology, Cincinnati Children’s Hospital Medical Center, USA
8Institute for Stem Cell Biology and Regenerative Medicine, Stanford University School of Medicine, USA
9Department of Applied Biological Science, Faculty of Science and Technology, Tokyo University of Science, Japan
10Division of Gene Expression Dynamics, Medical Institute of Bioregulation, Kyushu University, Japan
11Department of Pediatrics, University of Cincinnati College of Medicine, USA
12Center for Stem Cell and Organoid Medicine (CuSTOM), Cincinnati Children’s Hospital Medical Center, USA
13World Premier International Research Center Initiative Premium Research Institute for Human Metaverse Medicine (WPI-PRIMe), Osaka University, Japan
Related articles
Further information
Professor Takanori Takebe
Institute of Integrated Research, Institute of Science Tokyo
- ttakebe.ior@tmd.ac.jp
Contact
Public Relations Division, Institute of Science Tokyo
- Tel
- +81-3-5734-2975
- media@adm.isct.ac.jp